FAQ About Michael Faraday
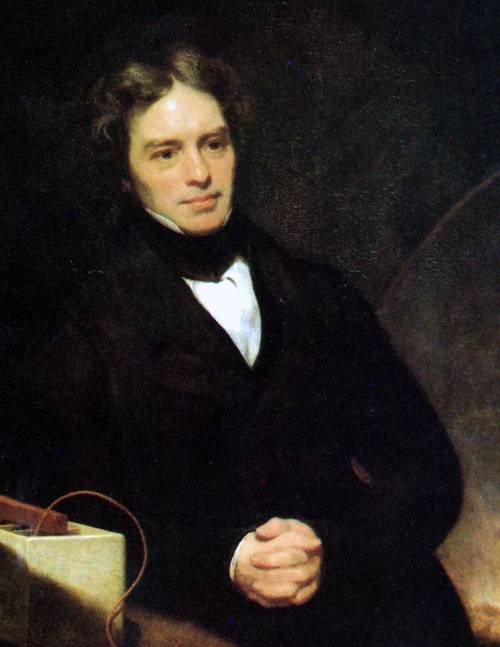
What are Faraday's laws of electrolysis?
Faraday’s laws of electrolysis are quantitative laws that describe the relationships between the amount of substance liberated at an electrode and the quantity of electricity passing through the electrode. The first law states that the mass of substances altered at an electrode during electrolysis is directly proportional to the amount of electricity that passes through the circuit. The second law states that the mass of substances altered by a given quantity of electricity is proportional to their equivalent weight.